Output: How Much Vapor Would a Bubbler Bubble if a Bubbler Bubbled Vapor?
The key thing we'd like to know about a bubbler is: how much vapor gets delivered? We can vary the temperature, pressure, and carrier gas flow. How do we translate these into an output flow or concentration?
The bubbler is modeled as being just a vessel at constant temperature with a known input flow of carrier gas, presumed not to react with the liquid or dissolve into it in significant amounts.
We make the usual idealizing assumptions:
- the "head region" is at equilibrium with the liquid:
- Pv = Pv, eq
- temperature of the liquid = set temperature
- the system is at steady state
- only vapor leaves the system (no splashing, kids!)
The partial pressure of the carrier must be equal to the difference between the pressure in the headspace and the equilibrium pressure of the vapor.
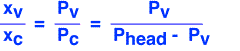
(Here "x" denotes mole fraction, v is vapor and c is carrier.) We assume that the outlet gas must have the same composition as the headspace gas, and that the gases are ideal so that the mole fractions of vapor and carrier have the same ratio as their partial pressures.
We can now solve for the output flow of vapor by realizing that every mole of carrier gas that goes in must come out (in steady state). The vapor out is equal to the product of carrier gas flow and the ratio of vapor molecules to carrier gas molecules, which we derived above.
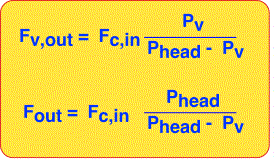
The total output flow is also easily obtained since it must be the sum of the carrier gas flow and vapor flow.
Let's look at a typical example for an APCVD process, here assuming that the head pressure is equal to the chamber pressure. We can see that a large flow of carrier is needed to deliver a small amount of vapor.
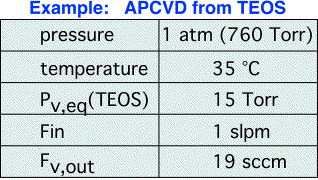
It should be apparent from the form of the equations that as we decrease the headspace pressure, the amount of vapor delivered increases. In the limit where the headspace pressure equals the equilibrium vapor pressure our equations say we should deliver an unlimited amount of vapor. (What really is happening here is of course that our assumptions would no longer hold, and a more complex model is required.) It is, however, very important to be cognizant of the general conclusion: bubblers are only useful for controlled vapor delivery into a chamber pressure higher than the equilibrium vapor pressure of the liquid! When this condition is violated, the amount of vapor delivered is no longer a simple function of the carrier flow, but depends on viscous drag between the bubbler and chamber.
Return to Tutorial Table of Contents
Book version of the CVD Tutorial
