Bubbler Problems and Challenges
Head Pressure
It is common to assume that the pressure in the headspace above the liquid is the same as the pressure in the reactor chamber. Is this likely to be true? Consider a fairly typical arrangement in which, for convenience and safety, the bubbler is placed in a cabinet or some other location remote from the reactor.
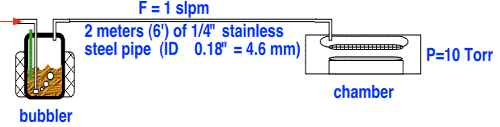
To calculate the pressure in the bubbler, we will assume Poiseuille flow in the tube, which as the reader will recall, is reasonable for slow flows and moderately long tubes.
The expression for the relationship between pressure and flow is:
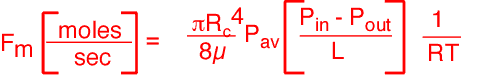
Using a viscosity of about 2.2x10-5 kg/m*s, we obtain a head pressure of about 28 Torr! This pressure is almost three times higher than that in the chamber; in consequence, the amount of vapor delivered to the chamber is considerably less than one would obtain using the chamber pressure as the head pressure.
In the other direction, as we noted previously, if the pressure in the chamber is less than the equilibrium vapor pressure of the vapor in question, our simple model fails. Not only does the model fail, the bubbler doesn't work very well, because vapor flows into the chamber in the absence of carrier gas, with a rate dependent on the viscous drag of the plumbing (which we typically didn't control precisely): that is, you may have little control over the amount of vapor delivered. This problem is particularly sneaky because for sufficiently high carrier flows (where the head pressure gets high enough to exceed the vapor pressure) the system is controllable and behaves roughly according to the simple models, but the threshold for controllable delivery depends on the plumbing drag and operating pressure and needs to be measured. In general it is a bad idea to use a bubbler to deliver vapor to a chamber at a total pressure below the vapor pressure in the bubbler source.
Temperature Control
A typical bubbler is a large vessel surrounded by a heater. Since bubbles keep the liquid agitated, the liquid itself may be reasonably isothermal; but the liquid temperature may differ considerably from the set temperature, due to e.g. transient cooling when carrier gas is turned on. Variations of 5°C over 1 hour in 280 ml of liquid are typical [Love et. al., J Cryst Crowth 129 p. 119 (1993)].
If the vessel is made very small to minimize characteristic time for heat transfer, it will need to be refilled frequently: variations in liquid level change temperature response and may affect vapor saturation in the headspace. The best way to run a bubbler is to have frequent small refills to avoid changes in level during operation, but the displacement of vapor due to the change in liquid level during the refill will induce a small transient change in the vapor delivery which must be dealt with.
If the liquid temperature is > ambient temperature, the plumbing lines and chamber wall must be heated to avoid condensation of the vapor. This is very difficult! Long thin pipes are NOT naturally isothermal: one thermocouple in 2 meters is not sufficient! It is very difficult to provide uniform heat delivery and achieve uniform heat loss along the length of pipe with e.g. heating tape and insulation. Better results can be obtained by placing all the plumbing in a heated isothermal box, but then one must deal with the fact that standard valves and mass flow controllers don't like to get very hot. MFC's with remoted electronics are available to allow operation to (at least) 80 °C.
Advanced integrated plumbing systems in which all the components are mounted to a backplane have become available in the last few years; however, these don't necessarily solve the problem. Temperature control of gas plumbing is difficult and requires careful measurements, heating arrangements, and insulation.
I Told You Kids To Stop Splashing!
Volume flow is greatly increased at low pressures, so the velocity of the bubbles goes up. At low operating pressures and high flows splashing can result, pushing liquid into the outlet.
Carrier Gas Purity
A large amount of carrier gas passes through a typical precursor during the bubbling operation:
1 liter of liquid TEOS = 4.8 moles
if bubbled at a mole fraction of 0.02 (from our APCVD example)
5450 liters of carrier gas are required!
Any contaminant in the carrier gas that reacts with the liquid (e.g. water vapor for TEOS) has lots of opportunity to work! This problem shows up as slow variations in film deposition rate, contamination in the film, and other nasty and hard-to-trace degradations of the process.
Carrier gas getters and purifiers of various kinds are commercially available. These should be used when the process or liquid is particularly sensitive to moisture or oxygen, common contaminants in such "inert" carriers as nitrogen or argon. An alternative approach is to purchase ultrapurified carriers. In both cases, it is important to make sure that the plumbing is leak-checked and thoroughly purged after each time a change is made or a reservoir replaced.
Return to Tutorial Table of Contents
Book version of the CVD Tutorial
