Chemical Vapor Deposition Basics
CVD is the formation of a film on a surface from a volatile precursor (vapor or gas), as a consequence of one or more chemical reactions which change the state of the precursor. Many different films can be deposited: elements and compounds, crystalline, polycrystalline, and amorphous. Most films can be deposited from several different precursor systems. Plasma discharges can be used to help things along, or the substrate and/or the gas can be heated or cooled.
Different deposition techniques, process conditions, and treatment after deposition produce films with differing characteristics, suitable for different applications. Each film has an optimal set of characterization techniques. Nevertheless, in every case, CVD processes must:
- provide a volatile precursor containing the constituents of the film
- transport that precursor to the deposition surface
- encourage or avoid reactions in the gas phase
- encourage surface reactions that form the film
- and do it rapidly, reproducibly, and uniformly for industrial applications.
Lingo Locquacity
Like every other specialized field, chemical vapor deposition has its own literature, filled with obscure terminology. An innocent attempt to ascertain how to deposit plasma silicon dioxide might leave you trying to decipher something like:
"N2O ... and Ar .. are introduced through a 2-cm ID sintered alumina tube which is wrapped with an 8-turn copper coil. RF power (13.5 MHz, 20 W) is used to excite a plasma within the tube. 2% SiH4/He ... is injected downstream of the plasma tube via a gas dispersal ring located 1 cm below the plasma tube port... The sample is heated radiatively from the back side by a tungsten halogen lamp... The substrate temperature and processing pressure were 300C and 300 mTorr, unless otherwise noted. Typically, the N2O and Ar flow rates were 20 sccm and 160 sccm respectively, and the 2% silane flow rate was 10 sccm..."
--from "Kinetics Investigation of Remote Plasma Enhanced Chemical Vapor Deposition of SiO2", C. Courtney and H. Lamb; Proc. XIII Int'l Conf on CVD, The Electrochemical Society Proceedings Volume 96-5 p. 177 (1996).
- Why use nitrous oxide and not molecular oxygen? What role does argon play?
- Why is RF power provided at 13.5 MHz?
- Is 20 watts a lot or a little?
- Why is silane (SiH4) used as a silicon source?
- How does radiative heating work?
- What happens at 300 C?
- What does a pressure of 300 mTorr imply for the plasma?
- Why do we care about the gas flow rates? what is an sccm anyway?
The same questions arise in a different context if you grab a commercial CVD reactor...

...and pull off the panels (with permission!) to see what’s inside:
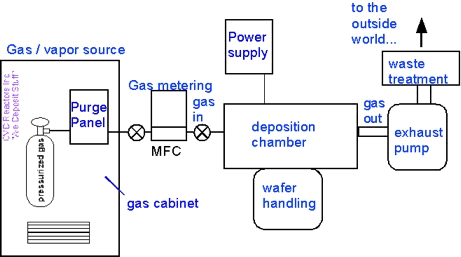
All this stuff supports what happens inside the deposition chamber:
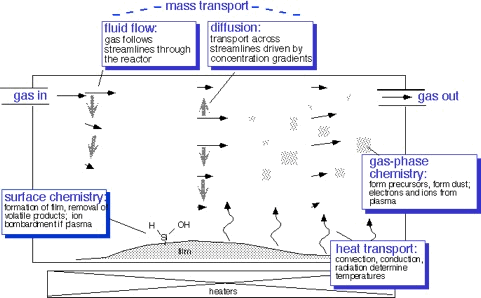
In order to understand how the process works, and thus why reactors are built the way they are, and what process engineers need to worry about, we need some understanding of all the things that go into making the process work
- Gas measurement and metering
- Transport of molecules by gas flow and diffusion
- Transport of heat by convection, conduction, and radiation
- Chemical reactions in the gas phase and at the surfaces
- Plasma formation and behavior
- Characterization of the resulting films
which are the topics of the remainder of this tutorial.
Return to Tutorial Table of Contents
Book version of the CVD Tutorial
